Which of the Following Does Not Describe a Polymer
Which of the following does not describe an oxidation reaction. The rest of the properties are given in option A B and C are true.

Polymer Description Examples Types Material Uses Facts Britannica
Correct option is D Polymers have very high molecular weight 10 310 7μ.

. Due to their broad spectrum of properties both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymer collapse in miscible good solvents is a generic phenomenon driven by. The Journal of chemical physics 2015.
Mean-field polymer theory does not describe polymer collapse transition in a mixture of two competing good solvents. The compound 6-aminohexanoic acid is used to make the condensation polymer nylon-6. Molecules are distributed over a range of molecular weights where the difference in.
Its nucleotide bases contain adenine uracil cytosine and guanine. The two backbones of the DNA molecule consists of. Polymers make up many of the materials in living organisms including for example proteins cellulose and nucleic acids.
Polymers are chemical compounds whose molecules are very large often resembling long chains made up of a seemingly endless series of interconnected links. Contains the adenine-guanine patterns D. The type of polymerization mechanism used depends on the type of functional groups attached to the reactants.
Polymers are used in a variety of everyday products such as plastics paints clothing and cosmetic products. Carbohydrates are also called saccharides and their monomers are. DNA is the genetic material not proteins.
Your industrial chemical is a polymer if it meets both of the following criteria. Which of the following does not describe the number of other atoms a carbon atom can be bonded to. The word Polymer is derived from two Greek words Poly that means many numerous and Mer which means units.
The conversion of Fe2 to Fe3. Polymer breakdown requires an input of free energy and involves the consumption of water. Hence they are called macromolecules.
DNA which has a double helix structure contains deoxyribose and phosphate backbone. Examples of synthetic polymers include PVC polyvinyl chloride polystyrene synthetic rubber silicone polyethylene neoprene and nylon. A polymer ˈ p ɒ l ɪ m ər.
32 Free Energy and Catalysis Part 1. Thermoset plastics are made from a liquid. What is this side reaction.
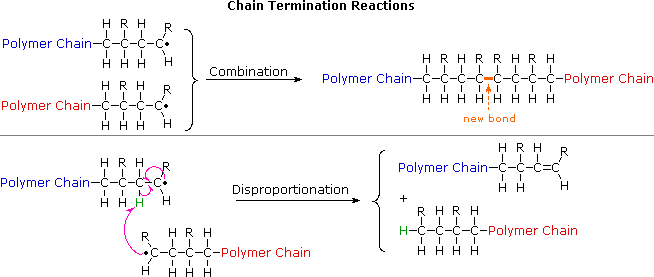
Polymerization is not always successful however because of a competing side reaction. Sets found in the same folder. Polymers are all created by the process of polymerization wherein their constituent elements called monomers are reacted together to form polymer chains ie 3-dimensional networks forming the polymer bonds.
Carbohydrates lipids proteins and nucleic acids. Polymers are both found in. The nitrogenous bases for DNA are adenine thymine cytosine and guanine.
In basic terms a polymer is a long-chain molecule that is composed of a large number of repeating units of identical structure. Polymerise ethene followed by hydration c H c H B c D. So the correct answer is D.
They are necessary for energy storage. The following diagram represents the structure of a possible polymer. The correct answer is option 1 ie.
Polymer any of a class of natural or synthetic substances composed of very large molecules called macromolecules that are multiples of simpler chemical units called monomers. Synthetic polymers are used to make plastics adhesives paints mechanical parts and many common objects. It is a definition to help you comply with our regulation.
Synthetic polymers may be grouped into two categories. On the other hand RNA has ribose and a phosphate backbone. Greek poly- many -mer part is a substance or material consisting of very large molecules or macromolecules composed of many repeating subunits.
Moreover they constitute the basis of such minerals as diamond quartz and. Starches in plants such as potatoes and maize. Cellulose in paper and trees.
These identical structures we understand as a unit made up of two or more molecules join together to form a. Which statement does not correctly describe the polymer PVC. Molecules composed of sugar monomers.
Solve any question of Polymers with-. There are four basic kinds of biological macromolecules. Natural polymers include.
OH OH OH OH OH OH cccc By which method might this polymer be made. A polymer is a large molecule that is made up of repeating sub-units connected to each other by chemical bonds. The size of these molecules as is explained in chemistry of industrial polymers is extraordinary ranging in the thousands and even millions of atomic mass units as opposed.
What did Hershey and Chases work show. A Salt is an Ionic compound which is not a Polymer. A only 3 B only 5 C only 4 D only 2.
Pitch also known as bitumen or tar Wool a protein made by animals Silk a protein made by insects Natural rubber and lacquer proteins from trees. Proteins such as hair nails tortoiseshell. Polymers range from familiar synthetic plastics such as polystyrene to.
These polymers are composed of different monomers and serve different functions.

Br On Twitter Science Biology Math Teacher Inspiration For Kids

Words Alone Can Not Describe The Fragrance When You Open This 5 Soap Giftbox This Beautiful Brushed Alu Handmade Natural Soaps Handmade Soap Bar Gifts

No comments for "Which of the Following Does Not Describe a Polymer"
Post a Comment